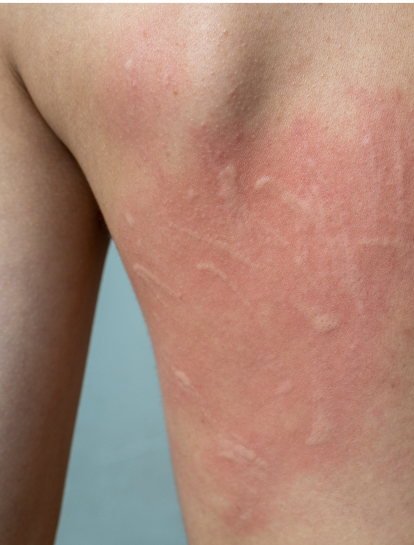
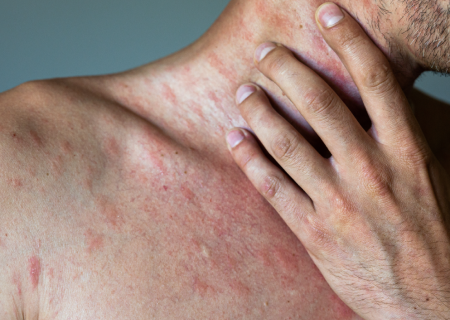
Hives, also known as urticaria, is a skin condition characterized by itchy bumps or welts on the skin. Chronic hives are welts that last for more than 6 weeks and often come back over months or years. The CALM clinical study is testing an investigational drug (EP262) to see if it will decrease hives in patients with Chronic Spontaneous Urticaria (CSU).
EP262 represents a new, targeted approach for the treatment of hives taken by mouth once a day.
If you experience chronic hives you may be interested in learning more about the CALM study.